Chasing shadows: Investigating X chromosome mediation in late-onset Alzheimer’s disease

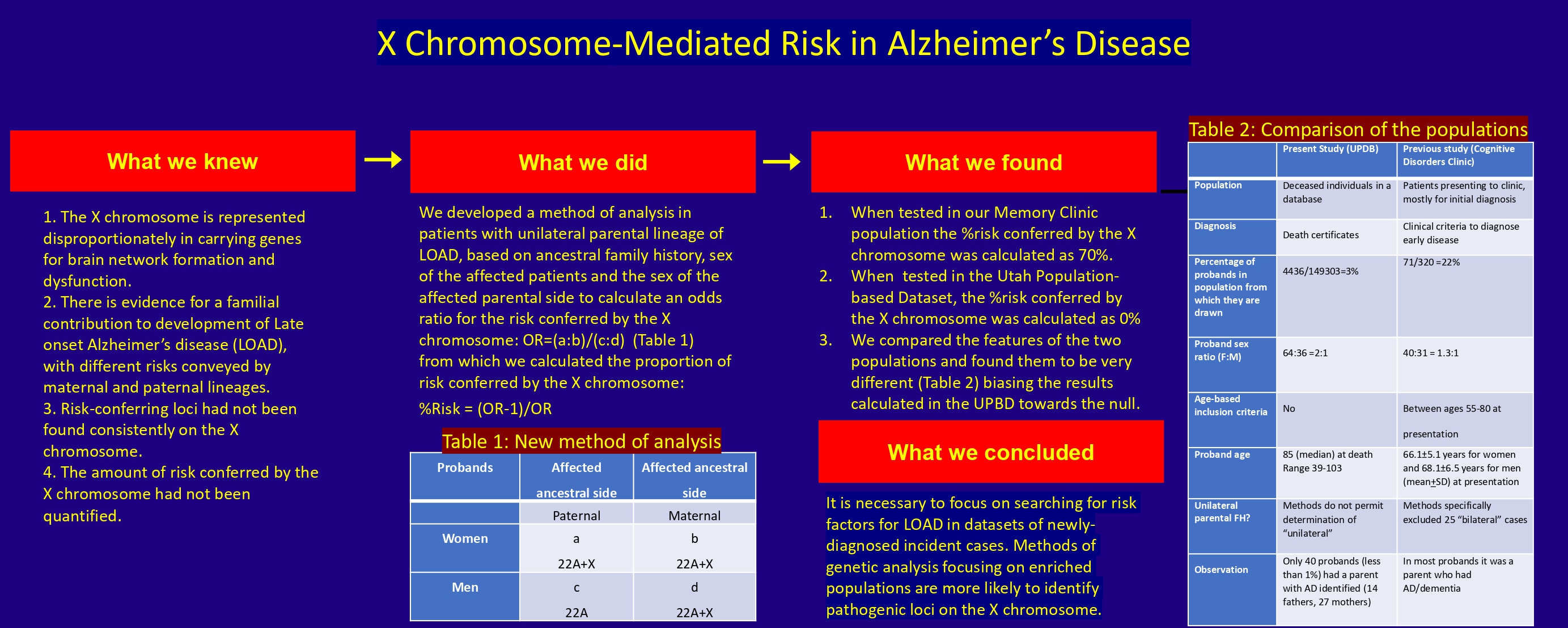
Alzheimer’s disease (AD) is a major cause of dementia. While maternal inheritance has been recognized for late-onset AD (LOAD), risk factors have not been identified consistently on the X chromosome. We recently developed a new method to identify an apparent risk of 70% mediated by the X chromosome in newly-presenting cognitive disorders clinic patients with amnestic mild cognitive impairment (aMCI) or early LOAD with unilateral parental lineage for AD or dementia. We sought to confirm our preliminary findings in the Utah Population Database (UPDB). We obtained previously published aggregate data on the risk of AD in the UPDB based on family history, stratified the data by the sex of the proband, and analyzed them using the new method. The X chromosome-attributable relative risk was estimated by calculating the following: Odds ratio (OR) = (women with paternal lineage: Women with maternal lineage)/(men with paternal lineage: Men with maternal lineage). The proportion of genetic risk attributable to the X chromosome is equal to (OR-1)/OR. The analysis did not reveal any risk mediated by the X chromosome, and the null result could be attributed to methodological limitations. Factors that impact the initial or early presentation (incidence) of LOAD, which are appropriate for consideration as risk factors, may not be detectable in a (prevalent) population of deceased individuals. Thus, epidemiological evidence for the contribution of the X chromosome to the development of LOAD will need to be sought prospectively in incident patient populations with newly diagnosed, biologically-confirmed aMCI or LOAD.
- Herbert LE, Weuve J, Scherr PA, Evans DA. Alzheimer disease in the United States (2010-2050) estimated using the 2010 census. Neurology. 2013;80:1778-1783. doi: 10.1212/WNL.0b013e31828726f5
- D’errico P, Meyer-Luehmann M. Mechanisms of pathogenic Tau and Aβ protein spreading in Alzheimer’s disease. Front Aging Neurosci. 2020;12:265. doi: 10.3389/fnagi.2020.00265
- Soreq L, Bird H, Mohamed W, Hardy J. Single-cell RNA sequencing analysis of human Alzheimer’s disease brain samples reveals neuronal and glial specific cells differential expression. PLoS One. 2023;18(2):e0277630. doi: 10.1371/journal.pone.0277630
- Bellenguez C, Grenier-Boley B, Lambert JC. Genetics of Alzheimer’s disease: Where we are, and where we are going. Curr Opin Neurobiol. 2020;61:40-48. doi: 10.1016/j.conb.2019.11.024
- Guerreiro R, Wojtas A, Bras J, et al. TREM2 variants in Alzheimer’s disease. N Engl J Med. 2013;368:117-127. doi: 10.1056/NEJMoa1211851
- Cannon-Albright LA, Foster NL, Schliep K, et al. Relative risk for Alzheimer disease based on complete family history. Neurology. 2019;92:e1745-e1753. doi: 10.1212/WNL.0000000000007231
- Edland SD, Silverman JM, Peskind ER, Tsuang D, Wijsman E, Morris JC. Increased risk of dementia in mothers of Alzheimer’s disease cases: Evidence for maternal inheritance. Neurology. 1996;47:254-246. doi: 10.1212/wnl.47.1.254
- Honea RA, Swerdlow RH, Vidoni ED, Burns JM. Progressive regional atrophy in normal adults with a maternal history of Alzheimer disease. Neurology. 2011;76:822-829. doi: 10.1212/WNL.0b013e31820e7b74
- Honea RA, Vidoni ED, Swerdlow RH, Burns JM, Alzheimer’s Disease Neuroimaging Initiative. Maternal family history is associated with Alzheimer’s disease biomarkers. J Alzheimers Dis. 2012;31:659-668. doi: 10.3233/JAD-2012-120676
- Roberts RO, Geda YE, Knopman DS, et al. The incidence of MCI differs by subtype and is higher in men: The mayo clinic study of aging. Neurology. 2012;78:342-351. doi: 10.1212/WNL.0b013e3182452862
- Mielke MM, Vemuri P, Rocca WA. Clinical epidemiology of Alzheimer’s disease: Assessing sex and gender differences. Clin Epidemiol. 2014;6:37-48. doi: 10.2147/CLEP.S37929
- Bajic VP, Essack M, Zivkovic L, et al. The X Files: “The mystery of X chromosome instability in Alzheimer’s disease”. Front Genet. 2020;10:1368. doi: 10.3389/fgene.2019.01368
- Yan Y, Wang X, Chaput D, et al. X-linked ubiquitin-specific peptidase 11 increases tauopathy vulnerability in women. Cell. 2022;185(21):3913-3930.e19. doi: 10.1016/j.cell.2022.09.002
- Eissman JM, Dumitrescu L, Mahoney ER, et al. Sex differences in the genetic architecture of cognitive resilience to Alzheimer’s disease. Brain. 2022;145(7):2541-2554. doi: 10.1093/brain/awac177
- Davis EJ, Solsberg CW, White CC, et al. Sex-specific association of the X chromosome with cognitive change and tau pathology in aging and Alzheimer disease. JAMA Neurol. 2021;78(10):1249-1254. doi: 10.1001/jamaneurol.2021.2806
- Davis EJ, Broestl L, Abdulai-Saiku S, et al. A second X chromosome contributes to resilience in a mouse model of Alzheimer’s disease. Sci Transl Med. 2020;12(558):eaaz5677. doi: 10.1126/scitranslmed.aaz5677
- Armon C, Wolfson S, Margalit R, et al. Estimating the X chromosome-mediated risk for developing Alzheimer’s disease. J Neurol. 2022;269:2479-2485. doi: 10.1007/s00415-021-10826-w
- Cannon-Albright LA. Utah family-based analysis: Past, present and future. Hum Hered. 2008;65:209-220. doi: 10.1159/000112368
- Kauwe JS, Ridge PG, Foster NL, Cannon-Albright LA. Strong evidence for a genetic contribution to late-onset Alzheimer’s disease mortality: A population-based study. PLoS One. 2013;8:e77087. doi: 10.1371/journal.pone.0077087
- Agresti A. Categorical Data Analysis. New York: Wiley; 1990.
- Furlan G, Galupa R. Mechanisms of choice in X-chromosome inactivation. Cells. 2022;11:535. doi: 10.3390/cells11030535
- García-González P, de Rojas I, Moreno-Grau S, et al. Mendelian randomisation confirms the role of Y-chromosome loss in Alzheimer’s disease aetiopathogenesis in men. Int J Mol Sci. 2023;24(2):898. doi: 10.3390/ijms24020898
- Rhie A, Nurk S, Cechova M, et al. The complete sequence of a human Y chromosome. Nature. 2023;621:344-354. doi: 10.1038/s41586-023-06457-y
- Armon C, Traynor BJ. High BMI is associated with low ALS risk: What does it mean? Neurology. 2019;93:189-191. doi: 10.1212/WNL.0000000000007852
- Brookmeyer R, Corrada MM, Curriero FC, Kawas C. Survival following a diagnosis of Alzheimer disease. Arch Neurol. 2002;59:1764-1767. doi: 10.1001/archneur.59.11.1764
- Complete Health Indicator Report of Life Expectancy at Birth. Utah Department of Health, Indicator-Based Information System for Public Health. Available from: https://ibis.health. utah.gov [Last accessed on 2022 Jul 17].
- Nelson PT, Dickson DW, Trojanowski JQ, et al. Limbic-predominant age-related TDP-43 encephalopathy (LATE): Consensus working group report. Brain. 2019;142:1503-1527. doi: 10.1093/brain/awz099
- Klyucherev TO, Olszewski P, Shalimova AA, et al. Advances in the development of new biomarkers for Alzheimer’s disease. Transl Neurodegener. 2022;11:25. doi: 10.1186/s40035-022-00296-z
- Printzlau F, Wolstencroft J, Skuse DH. Cognitive, behavioral, and neural consequences of sex chromosome aneuploidy. J Neurosci Res. 2017;95:311-319. doi: 10.1002/jnr.23951
- Wu H, Luo J, Yu H, et al. Cellular resolution maps of X chromosome inactivation: Implications for neural development, function, and disease. Neuron. 2014;81:103-119. doi: 10.1016/j.neuron.2013.10.051
- Ormond KE, Mortlock DP, Scholes DT, et al. Human germline genome editing. Am J Hum Genet. 2017;101:167-176. doi: 10.1016/j.ajhg.2017.06.012
- Komor AC, Badran AH, Liu DR. CRISPR-based technologies for the manipulation of eukaryotic genomes. Cell. 2017;168:20-36. doi: 10.1016/j.cell.2016.10.044
- Gupta RM, Musunuru K. Expanding the genetic editing tool kit: ZFNs, TALENs, and CRISPR-Cas9. J Clin Invest. 2014;124:4154-4161. doi: 10.1172/JCI72992
- Omura JD, McGuire LC, Patel R, et al. Modifiable risk factors for Alzheimer disease and related dementias among adults aged ≥45 Years - United States, 2019. MMWR Morb Mortal Wkly Rep. 2022;71:680-685. doi: 10.15585/mmwr.mm7120a2
- Power MC. Growing evidence links air pollution exposure to risk of Alzheimer’s disease and related dementia. Brain. 2020;143:8-10. doi: 10.1093/brain/awz396
- Van Dyck CH, Swanson CJ, Aisen A, et al. Lecanemab in early Alzheimer’s disease. N Engl J Med. 2023;388:9-21. doi: 10.1056/NEJMoa2212948
- Sims JR, Zimmer JA, Evans CD, et al. Donanemab in early symptomatic Alzheimer disease the TRAILBLAZER-ALZ 2 randomized clinical trial. JAMA. 2023;330:512-527. doi: 10.1001/jama.2023.13239

